|
Stem cell and Feeder Cell
Feeder Cell / Human Fetal Fibroblast (HFF)
Human Embryonic Stem Cell (hESC)
Induced Pluripotent Stem cell (iPSC)
Tissue engineering
Feeder Layer Cell Actions and Applications
Human Fetal Fibroblast (HFF)

|
Stem cells are biological cells that can differentiate into other
types of cells and can divide to produce more of the same
type of stem cells. They are found in multicellular organisms.
In mammals, there are two broad types of stem cells: embryonic stem cells, which are isolated
from the inner cell mass of blastocysts, and adult stem cells, which are found in various tissues. In adultorganisms, stem cells and progenitor cells act as a repair
system for the body, replenishing adult tissues. In a developing embryo, stem cells can differentiate into all the
specialized cells—ectoderm, endoderm and mesoderm (see induced pluripotent stem cells)—but
also maintain the normal turnover of regenerative organs, such as blood, skin,
or intestinal tissues.
https://en.wikipedia.org/wiki/Stem_cell |
Treatment
https://en.wikipedia.org/wiki/Stem_cell#Treatment
Diseases and conditions where stem cell treatment is being investigated include:
Research is underway to develop various sources for stem cells, and to apply
stem cell treatments for neurodegenerative diseases and
conditions, diabetes, heart disease, and other conditions.[91] Research
is also underway in generating organoids using stem cells, which would
allow for further understanding of human development, organogenesis, and modeling of human diseases.[92]
In more recent years, with the ability of scientists to isolate and culture embryonic stem cells, and with scientists'
growing ability to create stem cells using somatic cell nuclear transfer and techniques to
create induced pluripotent stem cells,
controversy has crept in, both related to abortion politics and to human cloning.
Hepatotoxicity and drug-induced
liver injury account for a substantial number of failures of new drugs in
development and market withdrawal, highlighting the need for screening assays
such as stem cell-derived hepatocyte-like cells, that are capable of detecting
toxicity early in the drug development process.[93]
Embryonic stem cell
https://en.wikipedia.org/wiki/Embryonic_stem_cell
|
Embryonic stem cells (ES cells or ESCs) are pluripotent stem cells derived
from the inner cell mass of
a blastocyst,
an early-stage pre-implantation embryo.[1][2] Human embryos reach
the blastocyst stage
4–5 days post fertilization,
at which time they consist of 50–150 cells. Isolating the embryoblast,
or inner cell mass (ICM)
results in destruction of the blastocyst, a process which raises ethical issues,
including whether or not embryos at the pre-implantation stage should have the
same moral considerations as embryos in the post-implantation stage of
development.[3][4] Researchers
are currently focusing heavily on the therapeutic potential of embryonic stem
cells, with clinical use being the goal for many labs. These cells are being
studied to be used as clinical therapies, models of genetic disorders,
and cellular/DNA repair. However, adverse effects in the research and clinical
processes have also been reported. |

|
Induced pluripotent stem cell
https://en.wikipedia.org/wiki/Induced_pluripotent_stem_cell |
|
|
Induced pluripotent stem cells (also known as iPS cells
or iPSCs) are a type of pluripotent stem cellthat can be generated directly from adult
cells. The iPSC technology was pioneered by Shinya Yamanaka’s lab in Kyoto, Japan, who showed in 2006 that the introduction of four
specific genes encoding transcription factors could
convert adult cells into pluripotent stem cells.[1] He was awarded the 2012 Nobel Prize along with Sir John Gurdon "for the discovery that
mature cells can be reprogrammed to become pluripotent." [2]
Pluripotent stem cells hold promise in the field of regenerative medicine.[3] Because they can propagate indefinitely, as well as give
rise to every other cell type in the body (such as neurons, heart, pancreatic,
and liver cells), they represent a single source of cells that could be used to
replace those lost to damage or disease.
|

|
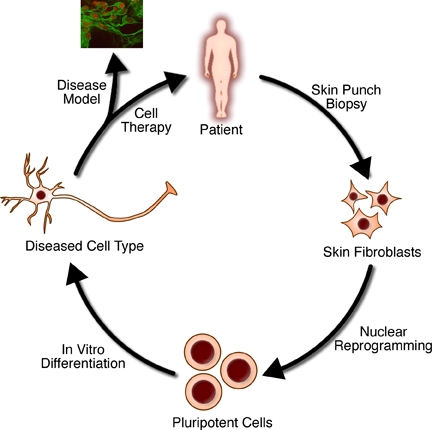
Tissue engineering
https://en.wikipedia.org/wiki/Tissue_engineering
|
Tissue engineering is the use of a combination of cells, engineering and materials methods, and suitable biochemical and physicochemical
factors to improve or replace biological tissues. Tissue engineering
involves the use of a tissue
scaffold for the formation of new viable tissue for a medical
purpose. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance
it can be considered as a field in its own. |
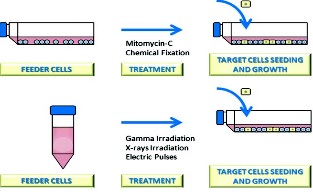
Feeder Layer Cell Actions and Applications
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4533020/
|
Cultures of growth-arrested feeder cells have been used for
years to promote cell proliferation, particularly with low-density inocula.
Basically, feeder cells consist in a layer of cells unable to divide, which
provides extracellular secretions to help another cell to proliferate. It
differs from a coculture system because only one cell type is capable to
proliferate. It is known that feeder cells support the growth of target cells by
releasing growth factors to the culture media, but this is not the only way that
feeder cells promote the growth of target cells. |
|
In this work, we discuss the different mechanisms of
action of feeder cells, tackling questions as to why for some cell cultures the
presence of feeder cell layers is mandatory, while in some other cases, the
growth of target cells can be achieved with just a conditioned medium. Different
treatments to avoid feeder cells to proliferate are revised, not only the
classical treatments as mitomycin or γ-irradiation but also the not so common
treatments as electric pulses or chemical fixation. Regenerative medicine has
been gaining importance in recent years as a discipline that moves biomedical
technology from the laboratory to the patients. In this context, human stem and
pluripotent cells play an important role, but the presence of feeder cells is
necessary for these progenitor cells to grow and differentiate. This review
addresses recent specific applications, including those associated to the growth
of embryonic and induced pluripotent stem cells. In addition, we have also dealt
with safety issues, including feeder cell sources, as major factors of concern
for clinical applications.
|
Human Fetal Fibroblast (HFF)
Why human feeder cells ?
Our solutions :
Human Fetal Fibroblast (HFF) Feeder Cell
The highest quality Human Feeder Cell of our team partner the
Capstone Biotek
|
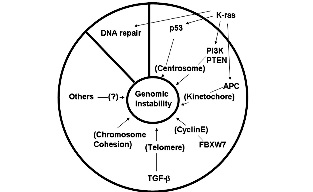
Lowest risk
Completely avoid the risk of xenogeneic introductions.
|
Compatability
Mitomycin-C treated human fetal fibroblasts (HFF) support human pluripotent cell
without introducing a second species to the culture conditions.
|
|
|
|
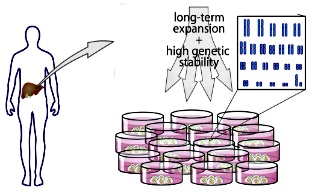
Long-term culture
Capable of maintaining pluripotency of stem cells (iPS/hESC) under long-term
culture.
https://www.cell.com/abstract/S0092-8674(14)01566-9
|
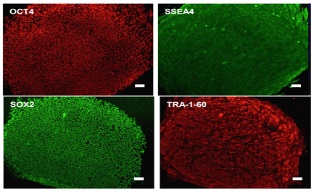
High quality
Our Human Fetal Fibroblast, HFFs, are tested comprehensively on human embryonic
stem cells and iPS cells to ensure consistent and robust performance.
SOX2, OCT4, SSEA4, and TRA-1-60 are pluripotency markers
|
|
|
Upgradable:
Different grades of feeder cells (Research grade/GMP grade) When the user
develops a product from academic research into a clinical human trial, It is
also stipulated in the regulations that the level of reagents used must also be
increased.
CBI's Research Grade - and Clinical-Grade HFFs are derived from the same cell
line, enabling our users for easily switching from Research Grade to
Clinical-Grade of HFF along the research phase develpoment while advancing cell
therapy products from research into clinical stage.
|
Clinical Grade :
Which are human fetal origin, history traceable, GMP/GTP compliance
|

|
Research Grade :
Which are human fetal origin, very affordable, could be easily switched to
clinical grade
|
CBI-HFF is the only source on the market that offers two grades of human feeder
cells.
|
|
|
Quality control:
Lot-to-lot consistency, negative for bacterial/fungal/mycoplasma/human pathogen
contamination.

|
Save time and money
Ready to use and for a reasonable price you can focus on your research instead
of daily routine cell expansion culture.
|
|
|
Safe handling
Our comprehensive contamination test include sterility, human pathogen, and
mycoplasma detection. This protect users and significantly minimizes the
contamination in your lab.
|
Create long term
cooperation with our customers in different product developmental stages.

|
|